When hydrogen and oxygen chemically react, water is produced and heat is generated.
2H2 + O2 → 2H2O + heat
A fuel cell is a device that converts this chemical energy into electrical energy.
+2+2+2+2+2+2
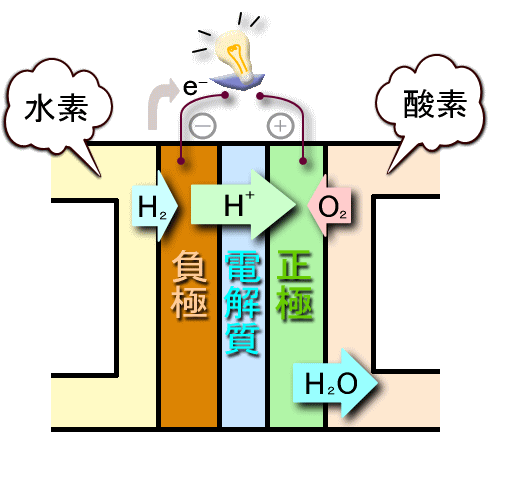
At the negative electrode (anode) of the polymer electrolyte fuel cell, electrons (e-) are emitted by oxidation of H2 in the fuel.
H2 → 2H++2e-
At the positive electrode (cathode), electrons passing through an external load reduce O2 to produce water.
1/2O2+2H++2e- → H2O-
As a clean power source with good energy efficiency, fuel cells are starting to be put to practical use in automobiles, private power generators, notebook computers, and so on.
Where can screen printing be used?
In polymer electrolyte fuel cells, screen printing is attracting attention as a technology for fixing noble metal catalysts on the positive and negative electrodes, and many studies have been presented at academic conferences.
In a solid oxide fuel cell, an oxygen electrode (positive electrode) and a fuel electrode (negative electrode) are fixed on both sides of the solid electrolyte. These electrodes act as (electronic) conductors and electrochemical reaction catalysts.
There is no technology superior to the screen printing method for thinly and evenly coating each electrode on the surface of the electrolyte.
The development of positive and negative electrode pastes for screen printing is being vigorously pursued.
+-+-+-+-+-+-

The solid electrolyte itself is a brittle sheet with a thickness of 1 mm or less.
The possibilities of screen printing are expanding in the field of fuel cells.
